Subtotal: $147.36
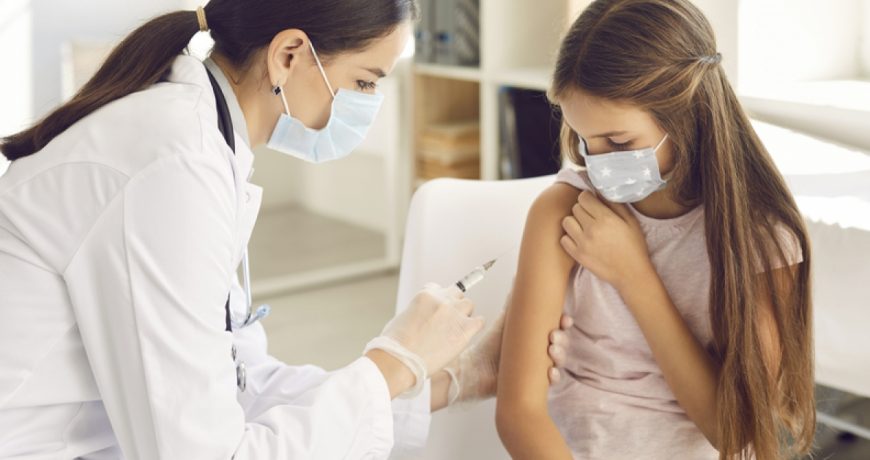
COVID-19 Boosters Authorized for Young Children
[ad_1]
(RxWiki News) The US Food and Drug Administration (FDA) has extended its authorization for two COVID-19 bivalent vaccines.
This authorizations expands the use of these vaccines to be used as boosters for a younger population than previously authorized.
A few months ago, the FDA authorized both the Moderna and Pfizer-BioNTech Bivalent COVID-19 vaccines for use as a booster dose for those:
- 18 years and older (Moderna COVID-19 Vaccine, Bivalent)
- 12 years and older (Pfizer-BioNTech COVID-19 Vaccine, Bivalent)
Now with this new authorization,
- The Moderna COVID-19 Vaccine, Bivalent is authorized for children as young as 6 years old
- The Pfizer-BioNTech COVID-19 Vaccine, Bivalent is authorized for children as young as young as 5 years old
Both of these vaccines are authorized to be given as a single booster dose at least two months after you have either:
- Completed the primary vaccination
- Have received a recent booster dose with a monovalent vaccine
Note: The Centers for Disease Control and Prevention (CDC) recommends that you get a bivalent booster even if you have gotten more than one original (monovalent) booster.
These “bivalent” vaccines are expected to offer better protection against the Omicron subvariants because they include the original virus strain plus a component in common between the omicron variants BA.4 and BA.5.
According to the CDC, the omicron variant BA.5 continues to cause the most cases of COVID-19 in the country.
“While it has largely been the case that COVID-19 tends to be less severe in children than adults, as the various waves of COVID-19 have occurred, more children have gotten sick with the disease and have been hospitalized,” said Dr. Peter Marks, director of the FDA’s Center for Biologics Evaluation and Research, in a press release. “Children may also experience long-term effects, even following initially mild disease. We encourage parents to consider primary vaccination for children and follow-up with an updated booster dose when eligible.”
For these two authorizations to happen, the FDA looked at the immune response and safety data seen in previous clinical studies.
As for side effects, those who receive either of these bivalent vaccines may experience similar side effects that have been reported by those who got the respective monovalent vaccine.
Speak with your healthcare provider if you have any questions about the available COVID-19 vaccines and booster recommendations.
[ad_2]
Source link

 #Semi-Automatic Digital Bpm Lot #
#Semi-Automatic Digital Bpm Lot #  Spec Mtch Bp Cuff & Sprge Blk Lot #
Spec Mtch Bp Cuff & Sprge Blk Lot #